Contents

 Gennova Biopharmaceuticals’ mRNA vaccine might just be a tad bit late to the vaccine party in the country
Gennova Biopharmaceuticals’ mRNA vaccine might just be a tad bit late to the vaccine party in the country
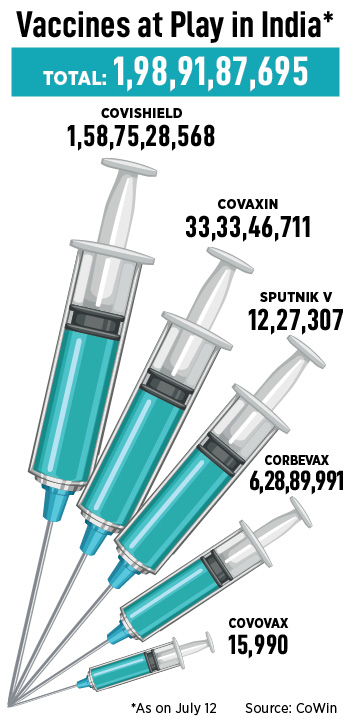
In India’s Covid-19 vaccination race, Gennova Biopharmaceuticals may perhaps just be a minimal late. Soon after all, the region has now accomplished 198 crore vaccinations, between the highest globally. Of these, 101 crore folks have received their 1st vaccine dose, though 92 crore persons have gained both of those. This is just about 70 p.c of India’s 138 crore populace.
Covishield, manufactured by Serum Institute of India (SII) in partnership with AstraZeneca, accounted for some 80 p.c of doses. Covaxin, produced by Hyderabad-based Bharat Biotech, accounted for a important part of the rest adopted by Organic E’s, Corbevax, and Russia’s Sputnik V.
The latest Covid-19 situations in India have not spiked to earlier amounts, with an normal of 16,000 conditions in a seven-day time period. Although the likelihood of a new virus variant stays, people’s life have almost swung again to usual, with attention fading from the pandemic.
This is why Gennova Biopharmaceuticals’ mRNA vaccine may just be a tad late to India’s vaccine celebration. The vaccine is the initially mRNA vaccine designed by an Indian enterprise, and is similar to globally administered vaccines by Pfizer-BioNTech and Moderna, therefore marking a milestone in India’s vaccine journey.
In contrast to vaccines that use a weakened or inactivated virus, mRNA vaccines use lab-made mRNA to create a protein, or even a piece of it, to cause an immune reaction. “Developing an unparalleled, storage-stable mRNA vaccine for India took time, as rolling out the liquid vaccine would not have been pertinent for India or our endeavours to democratise the mRNA vaccine globally,” states Samit Mehta, main operating officer, Gennova Biopharmaceuticals. “Thus, it took the time it did.”
The Pune-primarily based company’s vaccine, Gemcovac-19, is based on freeze-drying technological innovation that allows storage at 2 to 8 levels Celsius, compared to other mRNA vaccines that require sub-zero temperatures. “This will make it distinctive in its deployability in nations around the world like India,” provides Mehta. “However, we never believe we are late, provided the continued danger posed by Covid-19 and its distinctive variants. The versatility of the mRNA platform will enable us to develop a vaccine to overcome distinctive dominant strains in a pretty brief time.”
Gemcovac-19 employs a self-amplifying mRNA system, which provides it the benefit of a lower-dosing routine, says Gennova it has to be administered intramuscularly in two doses, 28 days aside. The mRNA know-how usually means it can be tweaked for rising variants, related to the DNA-dependent vaccine by Zydus Lifesciences.

“In the domestic market, the opportunity will be for the Omicron-distinct booster dose as very well as paediatric vaccination, since immunisation so considerably has been reduced,” Mehta states. “There is substantial potential in a number of emerging marketplaces.”
India’s Nationwide Technological Advisory Team on Immunisation’s (NTAGI) Covid working team is envisioned to examine information associated to Gemcovac-19 right after it acquired approvals for restricted use in emergency circumstances for 18-year-olds and previously mentioned from the Prescription drugs Controller General of India (DCGI). Gennova submitted their trial data in April.
“Well, they are definitely late in comparison to vaccines permitted in 2020-21,” suggests Satyajit Rath, emeritus professor, Indian Institute of Science Education and Investigation (IISER). “However, there is definitely area for new Covid-19 vaccines, together with as ‘cross-booster’ doses. Also, next-generation vaccines are pretty maybe wanted, and mRNA vaccines can be tweaked for this goal really fast, as well generate substantial stages of antibodies. And, of training course, regular refrigeration storage is an gain.”
 How Gennova upped its recreation
How Gennova upped its recreation
Gennova Prescribed drugs was established up in 2002, as a subsidiary of Pune-headquartered biotechnology business, Emcure Prescription drugs.
Founded in 1981 by Satish Mehta, the son of a pharma distributor, Emcure commenced as a agreement company for multinationals. Following far more than a decade, Mehta started marketing and advertising and distributing his very own branded generics. Right now, the company manufactures around 350 manufacturers, and operates five research and advancement (R&D) centres and 14 manufacturing services throughout India it has a existence in about 70 nations around the world.
A bulk of Emcure’s functions centre about persistent and sub-long-term therapies, which contribute virtually 65 p.c of its revenues. Its target parts consist of gynaecology, dermatology, anti-infectives, anti-retroviral, gastrointestinal, respiratory, oncology, cardiovascular, ache and natural vitamins. Last calendar year, Emcure posted revenues of Rs 6,054 crore.
In 2012, Gennova established its vaccine formulation and analysis centre, which was partly funded by Seattle-based non-earnings global wellness organisation Path’s Malaria Vaccine Initiative (MVI), which in flip was funded by the Monthly bill and Melinda Gates Foundation. Gennova’s vaccine enhancement features an antigen improvement programme and an adjuvant progress programme, in collaboration with the likes of USFDA and Johns Hopkins University.
With the Covid-19 outbreak, Gennova turned to its founded mRNA system to produce a vaccine, even as other pharma businesses moved speedily to possibly deal manufacture or acquire their own vaccines applying other systems. Although SII, India’s major vaccine maker, partnered with Uk-based AstraZeneca to manufacture Covishield, Bharat Biotech designed a vaccine utilizing a full-virion inactivated vero cell (useless coronaviruses).
“Gennova is the initially Indian firm to build an indigenous mRNA platform, and we have been performing on this platform for about four yrs,” Mehta says. “It is a pandemic-completely ready, illness-agnostic, swift-development platform technology.” Gennova’s function was partly funded by the Division of Biotechnology (DBT) in June 2020, underneath the Mission Covid Suraksha, a vaccine improvement programme allotted Rs 900 crore.
“At the onset of Covid-19, the DBT supplied portion of the seed funding of Rs 25 crore for building our vaccine manufacturing system,” claims Mehta. “Our inner accruals furnished the relaxation of the funding.” Right after its stage 1 scientific trials, Gennova received one more Rs 100 crore under Mission Covid Suraksha.
 Gennova is nevertheless to disclose its vaccine’s efficacy details, though it promises it’s related to previously permitted vaccines in India
Gennova is nevertheless to disclose its vaccine’s efficacy details, though it promises it’s related to previously permitted vaccines in India
Impression: Shutterstock
The mRNA vaccine
Although Gennova is nonetheless to price its vaccine, it states the pricing will be considerably additional competitive than its friends on a similar system.
One particular shot of the US-dependent Pfizer-BioNTech vaccine prices $23.15 in the European Union, while a person shot of Moderna’s vaccine charges $25.50, in accordance to revised supply contracts. Both equally vaccines have an efficacy amount of more than 94 percent. “This [pricing] can be decided only just after a dialogue with the federal government,” Mehta adds.
Gennova is nonetheless to disclose its vaccine’s efficacy info, despite the fact that it statements it’s similar to presently accepted vaccines in India. “The immune reaction examine was thorough and, in addition to measuring antibody concentrations, also assessed the neutralisation of the SARS-CoV-2 virus. Both equally the humoral and mobile responses were in contrast. Centered on the trial consequence, we can conclude that the immunogenicity of Gemcovac-19 was established to be equivalent.”
“The EUA for the mRNA vaccine involved many firsts,” clarifies Mehta. “The 1st time the group was generating a new entity, and it was the 1st time a product on the mRNA system was remaining examined in India. This kind of firsts typically confront their possess set of issues.”
Even as Gennova began producing its vaccine, it faced hiccups, like an US embargo on raw products at the peak of the pandemic. This meant turning to Emcure for uncooked components, together with enzymes required for building mRNA API.
Gennova has the potential to manufacture involving 40 lakh and 50 lakh vaccines a thirty day period, which can be speedily ramped up. “Gennova’s API capability is near to 500 million [50 crore] doses a calendar year, which can be doubled in a quick time. The fill-finish capacity is 200 million doses [20 crore] on a yearly basis and this much too can be ramped up at limited detect. A single of our crops in Pune has been repurposed for producing the Covid-19 vaccine,” claims Mehta.
Also read through: How can India put together for all the waves and variants in advance?
Can it perform in India nevertheless?
Whilst Gennova could be a late starter, Mehta says its system will assistance get ready for long run pandemics, which could contain mutations of the virus. “The creation of these an unprecedented sickness agnostic mRNA-technological know-how-primarily based facility empowers the world to be all set for foreseeable future pandemics,” he suggests.
“The mRNA vaccine is fairly substantial in terms of biotech industrial capacity,” says Rath of IISER. “mRNA vaccines are a lot quicker to tweak for up coming-generation vaccine progress, and they produce fairly superior stages of antibodies, while it is not distinct how they review with other vaccine platforms in conditions of period of responses. For that reason, mRNA vaccines are likely to be noticeable in the many years to come for a quantity of other diseases.”
There is also the probability that Gemcovac-19 could engage in a purpose amongst booster doses, although it is however to obtain needed approvals. Booster dose penetration is appreciably reduced, at less than 5 percent, in accordance to GlobalData, a London-based mostly consultancy firm.
 “While most of India’s eligible population have taken their original doses of Covid-19 vaccines, the mRNA system is possible to be an choice in the medium to lengthy time period as a booster dose,” mentioned Neha Myneni, pharma analyst, GlobalData, in a July 11 report. “Gemcovac-19 has not received approvals for use as a booster dose in India. That’s why, we count on small industry penetration as the proportion of the qualified populace however to get a Covid-19 vaccine is comparatively small.”
“While most of India’s eligible population have taken their original doses of Covid-19 vaccines, the mRNA system is possible to be an choice in the medium to lengthy time period as a booster dose,” mentioned Neha Myneni, pharma analyst, GlobalData, in a July 11 report. “Gemcovac-19 has not received approvals for use as a booster dose in India. That’s why, we count on small industry penetration as the proportion of the qualified populace however to get a Covid-19 vaccine is comparatively small.”
“Gemcovac-19’s approval definitely paves the way for more growth of property-developed mRNA vaccines for other conditions in India,” Myneni adds. “However, its results will very likely rely on the government’s conclusion to procure and deploy it, as most of the vaccinations in India are driven by the governing administration.”
Governing administration procurement is significant, as was evident from the lack of takers for Zydus’s ZyCov-D and Sputnik V, who had been early movers but have struggled considering that. At this time, as several as 40,530 vaccination websites are run by the government in comparison to 1,396 internet sites operate by personal establishments. With each other, the sector penetration of ZyCov-D and Sputnik V stand at less than 2 p.c.
Then there is the opportunity that Gemcovac-19 has in export marketplaces, specifically lessen and center-earnings international locations where by vaccine penetration proceeds to be reduced. “In this kind of circumstances, there could be unmet want for main vaccination and Gemcovac-19 can arise as the main solution, offered the vaccine’s medical success are built readily available,” states Myneni.
For Gennova, the journey has been a little bit extended. But it understands nicely that the fight is a lengthy-drawn a single and it has almost everything to enjoy for.
Check out our Monsoon discount rates on subscriptions, upto 50% off the web page rate, totally free digital entry with print. Use coupon code : MON2022P for print and MON2022D for electronic. Click in this article for aspects.